Common ELISA Problems and Solutions
Enzyme-linked immunosorbent assays (ELISA) are widely used tests to detect and quantify biological molecules. They are relatively simple and inexpensive to perform. However, one needs to fully understand the principles of an ELISA to properly interpret the results. As with any biological assay, things occasionally don’t go as planned. In this article, we will describe useful controls to include with each assay run and how to interpret results, and suggest solutions to try when the controls indicate a problem with the assay.
ELISAs come in many forms. For the purpose of this article, we will refer to a relatively straightforward and common ELISA format: the antigen capture.
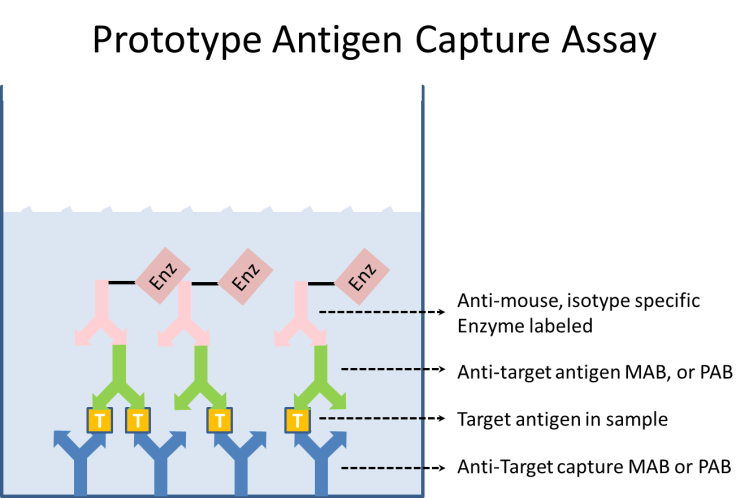
Each reagent is made in optimized buffers. One starts by adsorption of the anti-target capture monoclonal (MAB) to the microtiter plate. Excess MAB is washed out of the wells, and a blocking buffer is added to prevent further binding of any reagents to the plate itself. Typically there is a wash step between all reagent addition steps of the ELISA. A sample containing the target antigen is added and bound by the capture antibody. A second anti-target MAB, known as the detector, is added. This also binds to the target antigen. The detector MAB may be labeled in some way, or a third anti-mouse isotype-specific MAB which binds to the detector MAB is added to the wells. This third antibody will be labeled. Typically the label is an enzyme, but it may be any easily detectable entity. If an enzyme is used, then a substrate for that enzyme is added to the wells. The substrate is designed such that the action of the enzyme will produce a colorimetric change in the well, which may easily be measured at the proper wavelength of light by spectrographic instrumentation. This colorimetric change is usually reported as an optical density (OD, the log of the absorbance at the chosen wavelength). The optical density is then proportional to the amount of captured antigen in the sample.
Of course, controls should be performed along with the sample to ensure proper interpretation of the results for a given assay run. It is these controls that allow one to ensure that the assay is performed according to expectations. There is a short list of common controls typically included with each ELISA. Below we will define those controls and discuss what they can tell us about the performance of a given ELISA run.
The Blank (B)
The B control consists of a microtiter well or wells that are coated with the capture antibody and blocked with blocking buffer. These wells do not receive any sample or detector antibodies. The blank wells control for any variation, or contribution, of the plate itself to the measured OD. Expected values for the blank are quite low, approaching zero. Each assay typically has a few wells designated for the blank control. These wells may be placed adjacent to each other, or randomly across the plate to make the statisticians happy. If all goes well, then one can assume that the plates were manufactured properly and the blocking agent is performing as expected. However, if the blank well OD is higher than normally seen, the assay may have a problem. High ODs in the blank well may indicate a plate-washer problem. Further investigation of the plate-washing procedure, buffers, and instrumentation are warranted. The problem may be as simple as a clogged tube on the washer, especially if the problem is isolated to a single well. If the problem occurs in more than one well, a more thorough investigation of the wash process is needed. Once the washer checks out, then consideration of the substrate preparation should be investigated. Too much substrate can push the blank well ODs up as well.
Zero Concentration (ZC)
This ZC control is similar to the blank but contains all buffers and reagents from each step of the assay. The sample should contain only sample buffer with no target antigen. This is sometimes difficult to achieve, especially if one is measuring a common antigen that is found at measurable concentrations in the likely sample matrix. Sometimes one has to be creative to obtain or create a true zero concentration control. Once created or obtained, a ZC control allows one to determine the contribution of all reagents and buffers to our assay signal, absent any target antigen. Thus we have determined the true “background” of the assay.
If all goes well with the assay, one should expect to see OD values in the ZC wells that are only slightly higher than our blank wells. The actual values seen will likely vary among different assays for different target antigens. For each given assay, the control values should be consistent across runs. This control is also necessary if one wants to calculate a true limit of detection for an assay. Once the zero concentration is established, it serves as a benchmark for the sum of all reagents and buffers in the assay. Changes to the signal, either up or down, indicate a change to the assay that requires investigation. As with most ELISA performance issues, double-check the plate washer as described before. It cannot be stressed enough that proper care and maintenance of your plate washer will prevent many assay problems. However, once you have ruled out a plate-washer problem, it will be necessary to double-check reagent preparation, storage, and delivery. One needs to understand the stability profiles of all ELISA reagents. Adhering to the commercial kit guidelines usually prevents problems, but not always, so be aware.
Non-Specific Binding (NSB)
This is a variation on the blank and zero concentration wells. The goal is to further isolate the performance of assay reagents to ensure their proper function in the assay. For the NSB control, the wells are blocked as usual, but then blocking or wash buffer is added in place of the reagents at each step of the assay. The final addition of the labeled detector antibody is performed in the usual manner. Subsequent substrate development then occurs. This control allows one to assess the contribution of the labeled detector antibody to the overall OD signal of the assay. Desired OD results for NSB wells are slightly over the B control wells, but not over the ZC wells. Any differences in signal, up or down, can be attributed to the performance of the labeled detector antibody and may need to be investigated. Look carefully at reagent preparation and delivery. Improperly prepared detector antibody or substrate will easily affect this control and of course the rest of the assay.
Maximum Binding (B0)
We have been focusing on controls that look at the low end, or background, of the assay signal. Of equal importance is a good estimate of the maximum signal possible in the assay. This can be achieved by adding saturating amounts of sample to the capture assay, followed by saturating amounts of the labeled detector. After addition of substrate, a maximum colorimetric response will be determined.
A maximum binding control is necessary if one wants to subsequently calculate a % bound of sample. This calculation is performed when utilizing a competitive format antigen capture assay. In this case the labeled target antigen would compete with unlabeled from the sample. However, even without using the competitive format, it is a good idea to perform a maximum binding control. By using this control, one will know the upper limit of the signal generated by the assay. Depending on how the assay has been developed, values for such a control may actually exceed the ability of the instrument to measure OD. If this is the case, it may be necessary to cut back on the target antigen concentration until the OD is reduced to measurable levels. If the maximum binding OD values then drop, or even rise, one knows that there has been a change in the assay performance. As always, give the washer and the wash procedure a good review. After determining that the washer is working well, examine assay procedures and reagent preparation and delivery. Typically this control will pick up sample, substrate, or detector antibody issues. If a problem occurs, it is likely the B0 control will come in lower than expected, which may indicate a preparation or delivery problem in one or more of the three key reagents.
Summary
The use of the controls described above will help in determining the probable cause and hopefully lead to solutions to any problems encountered in the performance of an ELISA. In summary, there are really only a few things that can go wrong with an ELISA. As stressed above, the plate-wash instrumentation, buffers, and procedure are always likely causes of problems. Plate-wash problems frequently mimic reagent issues, so it is always wise to ensure that the washer is not the source of problems before pursuing other ends. Assuming the washer is not the problem, one typically can categorize the problems as follows: low or no signal, high background, inconsistent results between replicate samples or controls, or samples out of range (high or low).
If the entire plate is blank or has minimal signal, one can conclude that a procedural error has occurred or a key reagent was bad. Since the low signal is seen across the plate, it is likely that the labeled detector antibody was not added to the assay system. It is also possible that the enzyme is non-functional, but this is rare. A quick test for enzyme functionality is to add some correctly prepared enzyme labeled MAB directly to the substrate. The expected colorimetric change should occur rapidly. Another possibility is that the substrate is bad or was improperly prepared. Double-check procedures to make sure that the appropriate substrate buffer was used, as this may severely inhibit the expected color change. If you have an old lot of substrate, you can compare this to the new lot to ensure activity is relatively similar.
A high background is likely a reagent preparation problem. Too much labeled detector may cause this type of response in an assay. One has to double-check that the blocking step was also performed correctly. Was the right amount of block used in the right buffer?
Inconsistent results across replicate samples or controls are one of the most difficult issues to deal with. Assuming we have ruled out the plate washer, then one might have to consider a bad lot of plates or manufacturing issues. To rule out manufacturer problems, it may be necessary to test different kit lots. Be sure to track the lot numbers of the individual components of each test kit. Frequently, kit lots share the lot numbers of one or more components. In addition to bad plates, one has to look at the ability of the operator to pipette accurately. Small variations in pipetting across multiple reagents can produce results that will not pass quality control limits of variability.
Sometimes the controls all pass quality control criteria, but the samples themselves appear to be problematic. On occasion one may notice that the samples are exhibiting higher or lower than normal OD signals. Depending on the expected variability of samples, this can be difficult to notice. It is likely due to procedural issues such preparing the wrong sample dilution, using the wrong buffer, or pipetting the incorrect amount. One should also be aware of sample handling. While it is relatively easy to document sample handling in one’s own laboratory, it can be quite difficult to determine if problems have occurred somewhere else in the sample’s chain of custody. Be aware that sample handling is of the utmost importance for reliable results, both within one’s own laboratory and outside the laboratory.
Should your assay not behave as expected, the solutions discussed above will provide good starting points to quickly resolve your problems.
Written by James E. Drummond Ph.D